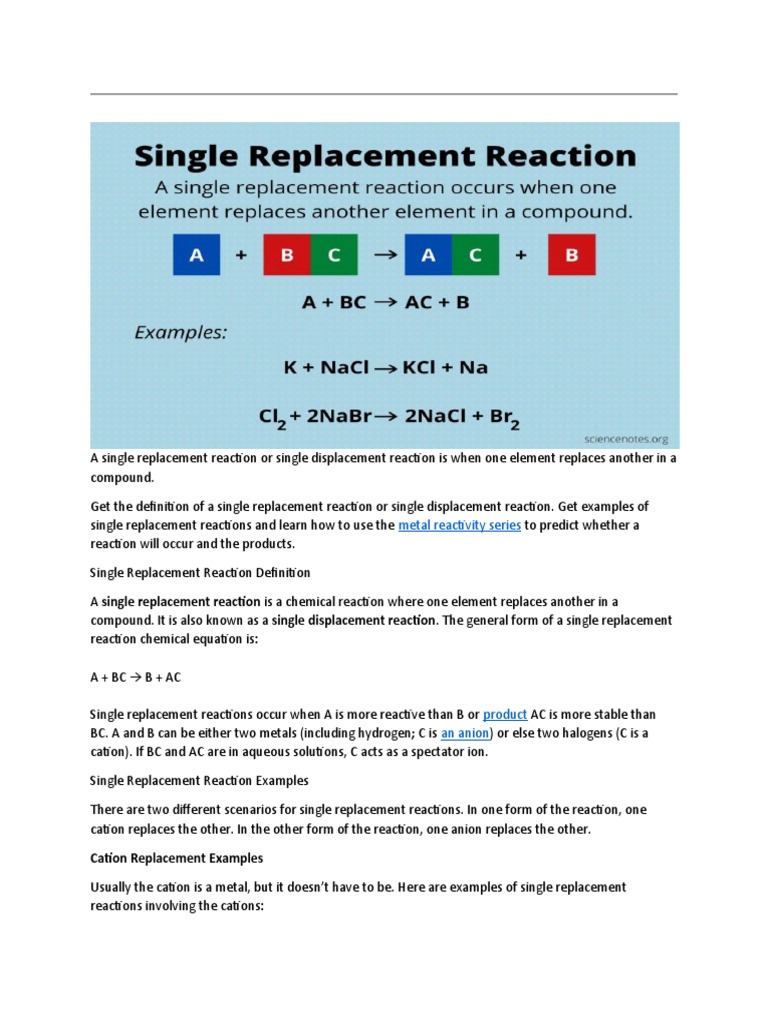
Single replacement reactions, often referred to as single displacement reactions, are a foundational concept in the world of chemistry. These reactions showcase how one element or ion can take the place of another element or ion in a compound, illustrating the dynamic nature of chemical interactions. This article delves into the intricacies of single replacement reactions by discussing their mechanisms, providing vivid examples, and highlighting their significance in both academic and practical realms.
To grasp the essence of single replacement reactions, one must first understand the underlying principles. In a single replacement reaction, an element reacts with a compound, leading to the substitution of one component of the compound by the element. The general formula for these reactions can be expressed as:
A + BC → AC + B
In this equation, A represents a reactive element, while BC signifies a compound composed of two elements. Upon reaction, A displaces B from the compound, resulting in a new compound AC and releasing the displaced element B. This displacement occurs due to differences in reactivity, which can be predicted using an activity series—a list of metals arranged in order of decreasing reactivity.
Common Observations in Single Replacement Reactions
One of the most captivating aspects of single replacement reactions is their frequent occurrence in everyday life. For instance, consider when zinc metal is placed in a solution of copper(II) sulfate. This striking visual demonstrates the reaction vividly. As zinc displaces copper—turning the solution from a vibrant blue toward a clear hue—one can witness the forming of copper metal as a precipitate. This tangible change not only affirms the principles of chemistry but also beckons observers to ponder the underlying forces at play.
Example: Zinc and Copper(II) Sulfate
The aforementioned reaction between zinc and copper(II) sulfate can be summarized as follows:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Here, zinc, a more reactive metal, actively displaces copper from copper(II) sulfate. The visual transformation captures attention as metallic copper precipitates out of the solution, revealing the intricate drama of atomic exchanges. This observation serves as an accessible entry point into the enchanting world of chemical reactions.
Mechanism of Reaction
To comprehend the mechanism in depth, one must delve into the concept of oxidation and reduction—commonly referred to as redox reactions. In single replacement reactions, the reactive element is oxidized, while the displaced element is reduced. In our zinc and copper(II) sulfate example, the zinc atom loses electrons, transitioning from the neutral zinc metal to zinc ions (Zn²⁺), thus undergoing oxidation:
Zn → Zn²⁺ + 2e⁻
Simultaneously, the copper ions (Cu²⁺) from the copper(II) sulfate gain those electrons, resulting in the reduction of copper:
Cu²⁺ + 2e⁻ → Cu
This elegant interplay of oxidation and reduction not only illustrates the conservation of charge but reinforces the interconnectedness of elements in a chemical reaction.
Real-World Applications
The significance of single replacement reactions extends beyond the confines of the laboratory. These reactions play a vital role in various industrial applications, technological advancements, and even environmental processes. For instance, in metallurgy, the extraction of metals from their ores often employs displacement reactions. A prime application is the extraction of iron through the reduction of iron(III) oxide (Fe₂O₃) using carbon:
Fe₂O₃ + 3C → 2Fe + 3CO
Here, carbon, a more reactive substance, displaces iron from its oxide, paving the way for the industrial synthesis of one of the most valuable metals.
Another quintessential application arises in the realm of batteries, where redox reactions drive the energy conversion processes. In electrochemical cells, single replacement reactions occur, enabling the storage and release of energy. This dynamic power harnesses the beauty of chemical reactivity, facilitating the modern conveniences we often take for granted.
Environmental Impact and Health Considerations
Though single replacement reactions are an accomplished feature of chemistry, they are not devoid of environmental implications. They can either generate beneficial or detrimental compounds, influencing ecological systems. For example, reactions involving heavy metals can lead to hazardous byproducts that pose risks to both health and the environment. Exploring such reactions encourages a conscientious approach within chemical research and industrial manufacturing, emphasizing sustainability and safety.
Conclusion
Single replacement chemical reactions serve as a compelling nexus of theoretical concepts and tangible phenomena in our daily lives. They illuminate the continuous dance of atoms and ions, showcasing the principles of reactivity, oxidation, and reduction. From vivid laboratory demonstrations to their real-world applications in metallurgy and energy storage, these reactions epitomize the fascinating interplay of chemistry, providing endless avenues for discovery and innovation. As our understanding deepens, the allure of single replacement reactions remains an enduring testament to the elegance of the natural world, inviting both curiosity and introspection into the science that shapes our universe.