Have you ever pondered the dazzling interplay of electrons that characterizes the world of chemistry? Imagine a scenario where two substances engage in a spirited dance, exchanging electrons and transitioning from one oxidation state to another like partners in a ballet. This elegant exchange is foundational to our understanding of chemical reactions, particularly redox reactions. So, what is the quintessential example of a redox reaction, and how can it challenge our perception of matter? Let’s embark on this journey of discovery.
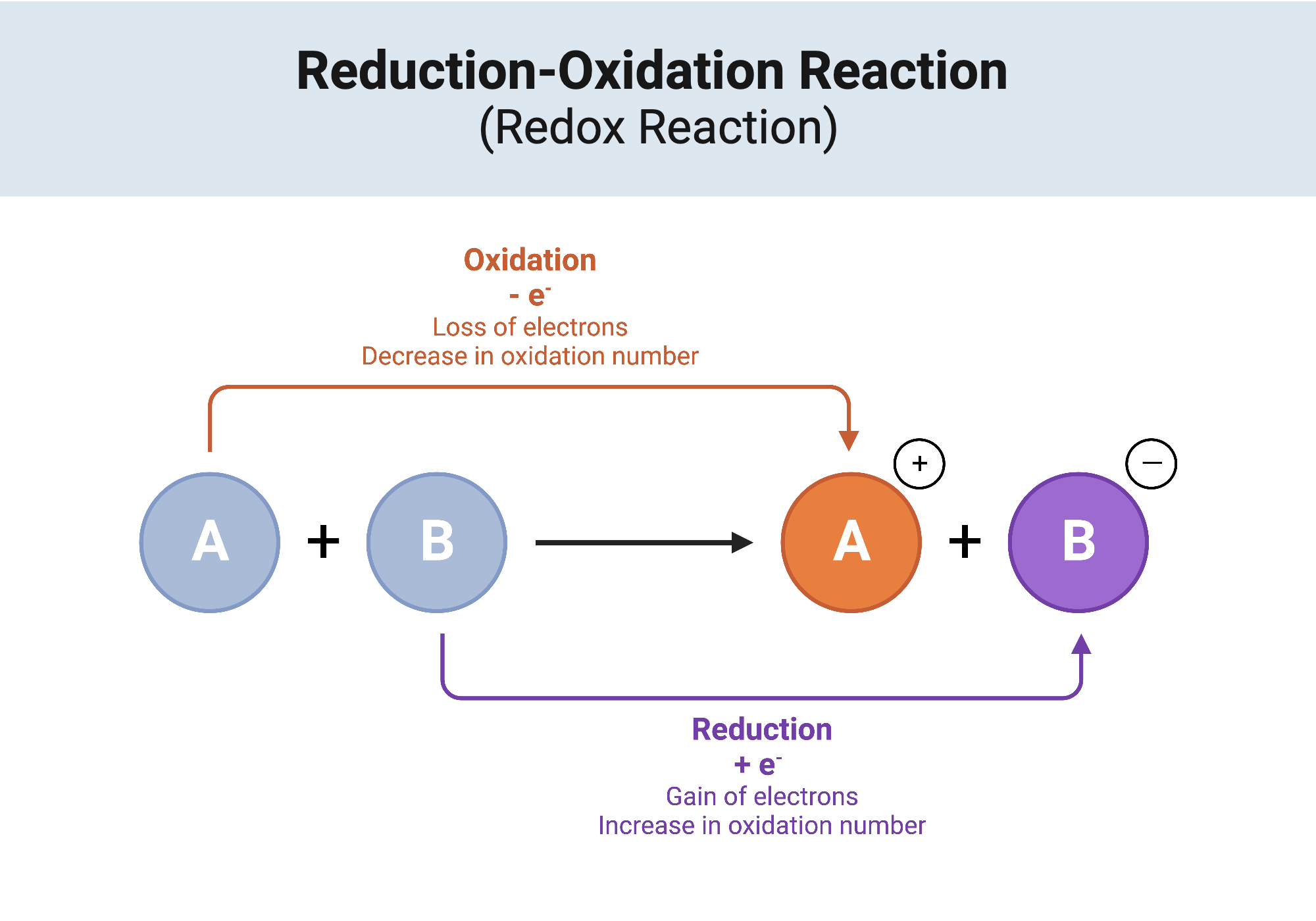
Redox, short for reduction-oxidation, refers to the coupled processes that dictate many chemical transformations. A redox reaction is characterized by the transfer of electrons between two species, leading to changes in their oxidation states. The species that loses electrons undergoes oxidation, while the one that gains electrons experiences reduction. In this intricate interplay, one might ask: what if a single electron could reshape an entire molecule? Let’s explore a classic illustration to solidify our understanding.
One of the most illustrative examples of a redox reaction is the transformation that occurs when iron rusts. The process of rust formation isn’t just a simple case of metal deteriorating; it showcases the fundamental principles of redox chemistry in action.
When iron (Fe) comes into contact with moisture and oxygen, it reacts to form iron oxide (Fe₂O₃), commonly known as rust. Initially, the iron atom is in a zero oxidation state. As it reacts with oxygen, it loses electrons and transforms into iron ions (Fe²⁺ or Fe³⁺), depending on the conditions. This loss signifies oxidation. The oxygen, on the other hand, serves as the electron acceptor, gaining these electrons and reducing its oxidation state. In the presence of water, the following simplified half-reactions occur:
Oxidation: Fe → Fe²⁺ + 2e⁻
Reduction: O₂ + 4e⁻ + 2H₂O → 4OH⁻Through these half-reactions, the transformation of elemental iron into rust illustrates the principles of conservation of mass and charge, showcasing how electron transfer facilitates the creation of a new compound. Nevertheless, I challenge you to consider: why does rust formation occur quicker in some environments than others? This reflects the nuanced interaction between external factors, such as humidity, temperature, and the presence of salts.
Aside from iron rusting, redox reactions are integral to several biological and industrial processes. For instance, in cellular respiration, glucose undergoes oxidation while oxygen is reduced, facilitating energy production in living organisms. This relationship not only emphasizes a fundamental aspect of life but also poses an intriguing question: how might the process of energy conversion in cells be influenced by dietary choices and environmental factors?
Now, let’s delve into the realm of galvanic cells, which harness the potential of redox reactions to generate electrical energy. A galvanic cell comprises two electrodes where distinct redox reactions occur. An exemplary galvanic cell is the Daniell cell, which consists of a zinc, a copper electrode, and two electrolyte solutions. In this setup, zinc undergoes oxidation while copper ions in solution are reduced.
The half-reactions in the Daniell cell can be written as follows:
Oxidation: Zn → Zn²⁺ + 2e⁻
Reduction: Cu²⁺ + 2e⁻ → CuAs these reactions transpire, electrons flow through the external circuit from the zinc electrode to the copper electrode, producing an electric current. This mechanism highlights how redox reactions can be harnessed to power devices, illustrating their significance in everyday technology.
However, the adventure doesn’t end here. Redox reactions also generate intriguing applications in various fields, including environmental science and analytical chemistry. For instance, the process of photoremediation employs organisms or plants to reduce environmental contaminants through redox reactions. This brings forth a thought-provoking question: how can our understanding of redox processes inform sustainable practices in pollution management?
Additionally, consider how redox reactions influence battery technology. Rechargeable batteries embody the essence of redox chemistry. During discharge, the chemical reactions release energy as electrons flow through an external circuit. Conversely, during charging, an external source reverses the reaction, restoring the original chemical compounds. This cyclical nature presents challenges and innovations for energy storage solutions.
Furthermore, the study of redox reactions extends into the realm of corrosion prevention. Protective coatings and sacrificial anodes are employed in industry to mitigate the adverse effects of oxidation. Here’s a challenge for the inquisitive mind: can you visualize a future where redox chemistry contributes to the development of even more advanced materials that resist corrosion?
In summary, the world of redox reactions is rich and multifaceted, manifesting in everyday occurrences such as rust and powering our devices through galvanic cells. These chemical processes not only allow us to convert and store energy but also challenge us to reconsider our relationship with nature and technology. As we continue to explore the boundaries of redox chemistry, it becomes increasingly apparent that the potential applications and implications are both vast and profound. So, as you contemplate the dynamics of electron exchange, remember that redox reactions are not merely a laboratory curiosity; they are integral to the fabric of our universe.