In the realm of organic chemistry, the concept of Lewis bases often emerges as a focal point of discussion. A Lewis base, by definition, is any species that donates an electron pair to form a covalent bond. This donation of electrons serves as a foundation for understanding a plethora of chemical reactions and interactions. Amidst this rich narrative, various examples of Lewis bases illustrate the profound implications of this concept in both theoretical and practical applications. This article delves into the captivating world of Lewis bases, elucidating their roles, properties, and significance in organic chemistry.
Understanding the Lewis Acid-Base Theory
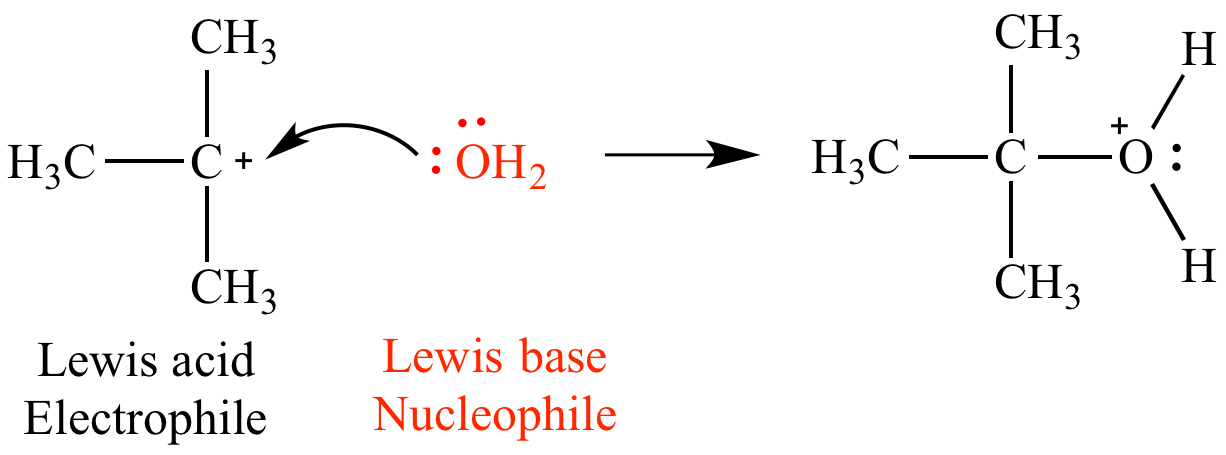
To better appreciate the concept of Lewis bases, it is essential to comprehend their relationship with Lewis acids. The Lewis acid-base theory, formulated by Gilbert N. Lewis, broadens the classical definitions provided by Brønsted and Lowry. In this framework, Lewis acids are electron pair acceptors, while Lewis bases are electron pair donors. The interaction between these two entities forms the crux of numerous chemical reactions, leading to the formation of adducts.
Common Examples of Lewis Bases
Among the myriad of Lewis bases, a few stand out prominently due to their unique structures and reactivity. Water (H2O), ammonia (NH3), and amines, in general, serve as quintessential representatives of Lewis bases.
Water, despite its ubiquitous presence, showcases a fascinating duality. It can act as both a Lewis acid and a Lewis base, depending on the context. When interacting with a stronger Lewis acid, such as sulfuric acid (H2SO4), water donates an electron pair, demonstrating its role as a Lewis base. This phenomenon hints at the intricate dance of electron sharing, which underpins much of organic reactivity.
Ammonia, with its lone pair of electrons on the nitrogen atom, exemplifies a traditional Lewis base. The nitrogen atom is electronegative, drawing electron density towards itself and allowing it to donate electron pairs in reactions, such as the formation of ammonium ions (NH4+) when interacting with protons (H+).
The Role of Hybrid Orbitals
The hybridization of atomic orbitals also significantly influences the behavior of Lewis bases. For instance, in the case of ammonia, the nitrogen atom undergoes sp3 hybridization, forming four equivalent sp3 hybrid orbitals. One of these orbitals contains a lone pair, making it a potent electron donor during reactions with Lewis acids. This intricate geometry not only affects bonding but also has implications for molecular interactions and properties.
Coordination Complexes
Lewis bases play a fundamental role in the formation of coordination complexes, which are pivotal in both natural and synthetic processes. In coordination complexes, a Lewis base coordinates with a metal ion (Lewis acid) through its electron pair, forming a complex that possesses unique chemical properties. Transition metals often serve as Lewis acids in these scenarios, leading to fascinating applications in catalysis and materials science.
A prime example is the coordination of ammonia to transition metal ions. These complexes are not just theoretical constructs; they hold immense significance in biological systems, such as hemoglobin’s iron center, which binds oxygen using similar principles of Lewis acid-base interactions. This connection showcases how Lewis bases are not merely abstract concepts but are integral components in essential biochemical processes.
Evaluating Lewis Basicity
Evaluating the strength of Lewis bases requires consideration of various factors, including electronegativity, steric hindrance, and the presence of electron-withdrawing or electron-donating groups. The ability of a Lewis base to donate its electron pair is significantly influenced by its overall molecular structure. For instance, in organic chemistry, substituting different groups on an amine can enhance or diminish its basicity, leading to a richer understanding of chemical behavior.
The comparative basicity of amines provides a striking illustration. Tertiary amines are often stronger Lewis bases than primary or secondary amines due to their lower steric hindrance, allowing for more effective orbital overlap with Lewis acids. This notion invites further scrutiny into the myriad factors that contribute to the reactivity of Lewis bases.
Applications of Lewis Bases in Organic Synthesis
The implications of Lewis bases extend into the domain of organic synthesis, where their electron-donating capabilities are harnessed for diverse reactions. For instance, Lewis bases can catalyze reactions such as Diels-Alder cycloadditions, facilitating the formation of complex cyclic structures. They are also instrumental in nucleophilic substitution reactions, where they attack electrophilic centers, paving the way for intricate molecular transformations.
Pushing boundaries, chemists exploit Lewis bases in modern synthetic methodologies. For example, the use of chiral Lewis bases has gained prominence in asymmetric synthesis, allowing for the selective formation of enantiomers. This endeavor not only deepens our understanding of reaction mechanisms but also enhances the efficiency of creating specific molecular architectures.
Conclusion: The Fascination with Lewis Bases
The allure of Lewis bases stems from their pivotal role in shaping chemical reactivity and their profound implications in both theoretical and practical domains. As we traverse through the landscape of organic chemistry, the contributions of Lewis bases illuminate the intricate connections between molecular interactions and macroscopic properties. In the end, the study of Lewis bases uncovers not only the elegance of chemical principles but also the unyielding quest for knowledge that fuels the world of science. Each example, from water to ammonia and beyond, contributes a brushstroke to the ambitious canvas of chemistry, revealing the intricate interplay of electrons that dictates the universe around us.