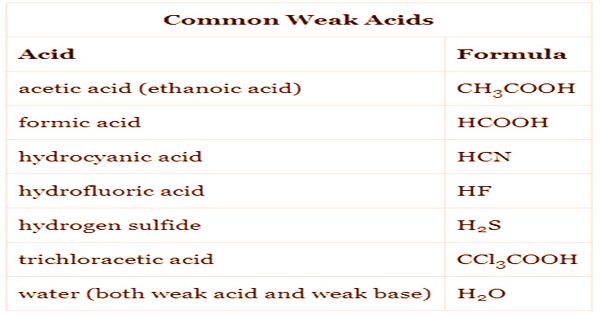
When you hear the term “weak acid,” what comes to mind? Perhaps you envision a laboratory setting, or maybe you think of a common household ingredient? While the phrase might invoke various images, the reality is often more complex than it appears. Weak acids are crucial players in numerous biological, industrial, and chemical processes. They promise fascinating phenomena and pose intriguing challenges when considered in practical applications.
This exploration delves into the depths of weak acids, exemplifying their unique properties, and shedding light on their widespread presence. Embarking on this journey, it’s essential to grasp what precisely qualifies a substance as a weak acid. Simply put, a weak acid is a substance that partially dissociates in a solution, releasing hydrogen ions while establishing a delicate equilibrium between the undissociated molecules and those that have dissociated.
Consider acetic acid, a quintessential example of a weak acid. Found in vinegar, which has been a staple in culinary practices for millennia, acetic acid possesses a structure that allows it to exhibit both acidic and aromatic properties. This duality renders it captivating, not only for its flavor-enhancing qualities but also for its applications in food preservation and fermentation. So, why is acetic acid classified as a weak acid?
Unlike strong acids—such as hydrochloric acid, which dissociates entirely in water—acetic acid’s dissociation is not absolute. In a typical vinegar solution, only a fraction of the acetic acid molecules release their hydrogen ions. This limited dissociation accounts for the moderate acidity of vinegar, which typically hovers around a pH of 2.5 to 3.0. The balance between the dissociated and undissociated forms fosters a more nuanced interaction with biological systems, influencing taste, metabolism, and even cellular functions.
The quintessential nature of weak acids can lead to various practical applications, but it also introduces challenges. Imagine a scenario where one attempts to craft the perfect salad dressing, only to be perplexed by the variances in flavor and acidity. The interplay of weak acid concentrations, their dissociation equilibrium, and the presence of additional ingredients can create unexpected results. How does one navigate these challenges to achieve the desired taste profile?
Above all, understanding the properties of weak acids like acetic acid reveals their multifaceted applications. In the realm of food science, acetic acid’s presence extends beyond mere flavoring; it plays a vital role in the fermentation of pickles, allowing for the safe preservation of vegetables by creating an acidic environment that deters harmful bacteria. This process highlights the synergy between weak acids and microbial growth, an essential facet of gastronomy.
Furthermore, weak acids are not limited to culinary delights. In biological contexts, they hold immense significance. Lactic acid, another notable weak acid, emerges during anaerobic respiration in muscles, resulting in the phenomenon commonly known as muscle fatigue. As the body undergoes strenuous activities, lactate accumulates, signifying a critical threshold of energy production. The intriguing interplay of lactic acid and muscle performance continues to intrigue researchers and athletes alike. How can one tap into the power of this weak acid for optimal physical performance?
Continuing on this odyssey through the world of weak acids, one cannot overlook citric acid, prominently found in citrus fruits. Citric acid demonstrates a trifecta of flavor—providing tartness, enhancing preservation, and acting as an essential intermediate in metabolic pathways (such as the Krebs cycle). Its contribution to the food industry extends to soft drinks, candies, and any number of diverse products. However, as we indulge in these flavors, it’s prudent to consider how citric acid can impact our health, especially in excessive amounts. Are we mixing pleasure with peril when we consume fruits loaded with citric acid?
As we traverse deeper into the properties of weak acids, we unravel layers of complexity and interrelations between various compounds and their environments. For instance, consider the common occurrence of formic acid, predominantly found in ant venom. Its role as a defensive agent illustrates that even the smallest concentrated solution can induce a sensation far beyond its volume. Encountering a swarm of ants, one might ponder the balance of strength and weakness present in their biochemistry. What can these small yet potent creatures teach us about the potency of weak acids?
The educational potential of weak acids extends beyond chemistry class. Their significance permeates numerous disciplines, from environmental science to medicine. Phosphoric acid, another weak acid, finds its niche in promoting dental health, despite its reputation as a sugary drink ingredient that can erode tooth enamel. This duality raises essential questions about responsibility and awareness regarding dietary choices infused with such weak acids. How might the intertwined fates of weak acids and human health empower us to make informed decisions?
In environmental contexts, weak acids also contribute to the formation of acid rain, an issue that emerges from human activities leading to sulfur dioxide and nitrogen oxides in the atmosphere. This phenomenon, while often overlooked, underscores the interconnectedness of weak acids within our ecosystem. Understanding their roles in climate dynamics may foster more significant environmental advocacy and responsible practices among individuals.
In conclusion, the world of weak acids offers a tapestry woven from culinary delights, biological intricacies, and environmental concerns. From the kitchen to the natural world, their influence permeates our lives, urging us to explore and understand their multifaceted roles. As we consider the delicate balance between their benefits and challenges, we are reminded that knowledge is the key. So, the next time acetic acid, citric acid, or lactic acid crosses your mind—or your palate—ask yourself: What stories do these compounds reveal about the chemistry that governs both nature and humanity?