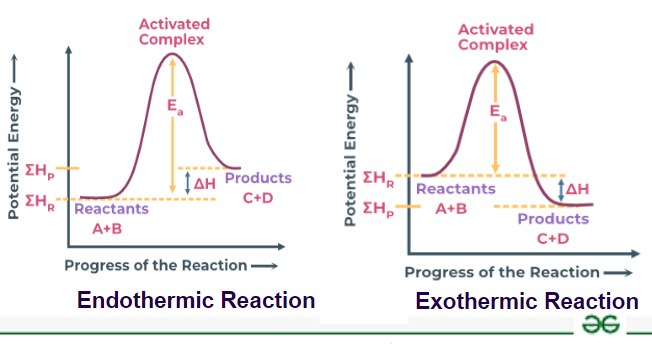
Exothermic processes are an integral part of our daily lives, occurring in various settings, ranging from the commonplace to the profoundly complex. The term “exothermic” itself derives from the Greek words “exo,” meaning outside, and “therm,” meaning heat. Essentially, these are reactions that release heat to their surroundings, often leading to a perceptible change in temperature. Let’s delve into various fascinating examples of exothermic processes, exploring how they play a pivotal role in nature, industry, and even our own culinary adventures.
One of the most ubiquitous examples of an exothermic reaction is the combustion of fossil fuels. This is perhaps the most familiar and widely recognized process, observable in our everyday activities. When we ignite gasoline in our cars, the hydrocarbon molecules react vigorously with oxygen in the air, producing carbon dioxide, water, and a significant amount of heat. This reaction is not merely a fuel-generating mechanism; it intricately intertwines with the principles of thermodynamics. The energy released facilitates motion, powering vehicles, and simultaneously heating the exhaust gases as they exit the engine. The efficiency of this energy transfer is a marvel of engineering, continuously optimized to reduce waste and enhance performance.
In the realm of nature, the process of cellular respiration showcases another exothermic reaction. Within the cells of living organisms, glucose reacts with oxygen in a meticulous series of biochemical reactions. The result? Energy! This energy is indispensable for maintaining cellular functions, including growth, reproduction, and homeostasis. When you consider the sheer volume of this reaction occurring within our bodies, it is nothing short of astonishing. It’s a constant, dynamic exchange, providing the energy we need to thrive. The warmth you feel after engaging in a physical activity is a testament to this biochemical frenzy, showcasing the release of heat as your cells metabolize nutrients.
On a grander scale, the process of nuclear fission represents one of the most powerful exothermic reactions known to mankind. In this process, the nucleus of a heavy atom, such as Uranium-235, splits into smaller nuclei, releasing a formidable amount of energy in the process. This exothermic reaction is the cornerstone of nuclear power plants, where the heat generated is harnessed to produce steam, which then drives turbines to generate electricity. Though it offers a significant output of energy, it is a double-edged sword, necessitating stringent safety measures and responsible management of radioactive materials. The phenomenal energy yield and the challenges it presents evoke a profound fascination, highlighting humanity’s quest for sustainable energy solutions.
In our kitchens, exothermic reactions can also be observed during food preparation. For example, the process of baking bread incorporates exothermic reactions between yeast and sugars. As yeast ferments, it metabolizes sugars and produces carbon dioxide and alcohol, along with heat as a byproduct. This heat contributes to the rise of the dough, making bread fluffy and delectable. The sensory experience of kneading dough and savoring the aroma of freshly baked bread is not only a culinary delight but also a reflection of the intricate biochemical transformations taking place. This simple yet profound act of making bread is indeed an exemplar of how exothermic processes interweave with culture and tradition.
Moreover, the phenomenon of thermophilic bacteria in hot springs embodies nature’s ingenuity through exothermic processes. These specialized organisms thrive in extreme environments, deriving their energy from sulfur or methane in an energy-releasing reaction that generates significant heat. The vibrant colors and unique ecosystems surrounding these hot springs captivate biologists and tourists alike. The lush microbial life in these harsh conditions illustrates the astonishing adaptability of life forms on Earth, producing a setting that is both beautiful and scientifically intriguing.
Furthermore, exothermic reactions find application in the production of self-heating cans, an innovation that allows for on-the-go consumption of beverages at ideal temperatures. By initiating a chemical reaction with a simple push or pull, these cans heat their contents without the need for an external heat source. This application of chemistry in consumer products demonstrates how exothermic processes can enhance our convenience and enjoyment of everyday life. The ingenuity behind this technology serves as a reminder of the hidden science at play in our daily choices.
Transitioning to a broader perspective, exothermic reactions have profound implications in environmental science and climate change discussions. The combustion of fossil fuels, while generating necessary heat and energy, also releases greenhouse gases, which contribute to global warming. This complex relationship between energy production and environmental impact underscores the critical need for sustainable energy solutions. Thus, understanding exothermic processes is essential not only in appreciating their immediate benefits but also in contemplating their long-term effects on our planet.
In conclusion, exothermic processes are far more than mere chemical reactions; they are the lifeblood of our energy systems, the foundation of sustenance, and the engines driving our technological advancements. Exploring the various manifestations of these reactions—from the combustion of fuels to biochemical processes in our bodies and even technological applications—invites us to marvel at the intricate interplay of heat, energy, and the natural environment. As we continue to navigate the challenges of the modern world, a deeper understanding of exothermic reactions may guide us toward more sustainable and innovative solutions for the future.