Have you ever wondered how table salt, that common seasoning gracing nearly every meal, comes into existence? What if I told you that this unassuming compound is a prime example of a fascinating phenomenon known as ionic bonding? Let’s embark on a journey to demystify the intricacies of ionic bonds and discover their pivotal role in the chemistry of the world around us.
At its core, an ionic bond is a type of chemical bond that arises from the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This interaction occurs as a result of the transfer of electrons from one atom to another. When a metal atom, which tends to lose electrons, interacts with a non-metal atom, which tends to gain electrons, an ionic bond is formed, creating a stable compound. But how does this process unfold, and what challenges does it pose?
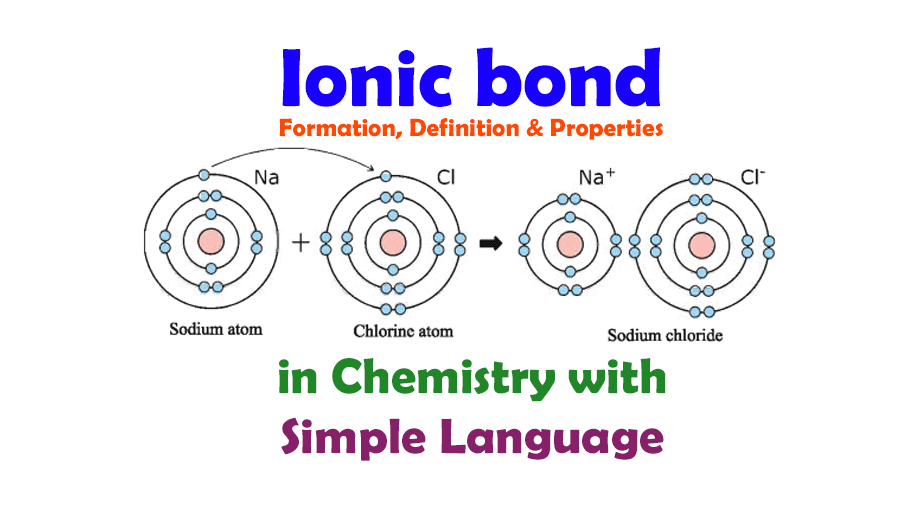
To elucidate the process of ionic bond formation, let’s examine two archetypical elements: sodium (Na) and chlorine (Cl). Sodium, a highly reactive alkali metal, possesses one loosely held outermost electron in its electron shell. By contrast, chlorine, a halogen, has seven electrons in its outer shell and requires one additional electron to achieve a stable octet configuration. It’s in this dynamic that a curious dance of atoms begins.
When sodium and chlorine come into proximity, sodium readily relinquishes its outer electron. This loss transforms sodium into a positively charged ion, known as a cation, because it now has an excess of protons compared to electrons. Chlorine, on the other hand, joyfully welcomes this wayward electron, converting itself into a negatively charged ion, or an anion. This newfound charge disparity—sodium with a +1 charge and chlorine with a -1 charge—sets the stage for the establishment of an ionic bond. But, why does this happen? What’s the underlying drive?
The propensity for atoms to achieve full outer electron shells drives this electron transfer. The quest for stability propels these elements into their respective roles: one give, one take. The moment sodium loses its electron and chlorine accepts it, the two ions become magnetically attracted to one another, resulting in the formation of sodium chloride (NaCl), or table salt. In essence, this interaction symbolizes a fundamental truth in nature: opposites attract.
Once formed, ionic compounds such as NaCl exhibit distinct properties, revealing the influence of their ionic bonds. Typically, ionic compounds crystallize into a lattice structure, where each sodium ion is surrounded by six chloride ions and vice versa. This geometric arrangement not only maximizes the attractions between the ions but also contributes to the unique physical properties of ionic compounds. Together, these ions create a sturdy, rigid structure that possesses a high melting point, generally requiring substantial energy to disrupt the order.
But could there be challenges associated with these transformations? Yes, indeed. While the process may seem straightforward at first glance, several factors can affect ionic bond formation. For instance, the presence of impurities or competing ions can hinder the purification of products resulting from ionic bonding. Moreover, environmental variables such as temperature and pressure can alter an ionic compound’s stability and solubility. In laboratories or on industrial scales, scientists must navigate these challenges to optimize yield and purity.
The wonder of ionic bonds extends beyond simple table salt. They play critical roles in biological systems as well. An intriguing example can be found in the role of sodium and potassium ions in cellular functions. Cells rely on the precise regulation of ionic concentrations to maintain electrical gradients across their membranes, fueling processes such as nerve impulse transmission and muscle contraction. Here, ionic bonds foster not only chemical stability but also the dynamic processes vital for life. Isn’t it remarkable how something as basic as an ionic bond can underpin complex biological systems?
Additionally, ionic compounds find extensive applications in various industries. For example, the medicinal realm utilizes ionic compounds in pharmaceuticals for delivering drug ions to targeted locations in the body. These interactions are crucial in ensuring the efficacy and safety of medications. The world of materials science also exploits the properties of ionic bonds, where ionic compounds serve as essential components in batteries, superconductors, and catalysts. The versatility of ionic bonds exemplifies their importance, transcending mere academic curiosity.
As we unravel the complexities of ionic bonding, it becomes apparent that these chemical interactions are not only foundational to chemistry but are also deeply interwoven into the fabric of our daily lives. Whether it’s seasoning our food or allowing our cells to communicate, ionic bonds are everywhere—molding the universe in subtle yet profound ways.
So, next time you sprinkle a dash of salt on your meal or marvel at the functioning of your body, take a moment to appreciate the role of ionic bonds. They symbolize the remarkable interplay of elements and serve as a reminder of the hidden complexities that lie beneath even the simplest aspects of our world. The challenge lies in recognizing and appreciating the science that exists all around us, waiting to be discovered.