In the grand tapestry of chemistry, covalent bonds stand as remarkable threads weaving together atoms, creating intricate structures and compounds that manifest vividly in our day-to-day lives. Much like a skilled artisan molding clay into unique shapes, atoms engage in the delicate dance of sharing electrons, crafting compounds that are not only functional but also beautifully complex. This article will navigate through the captivating world of covalent bond compounds, exposing a few exemplary stars that capture the essence and utility of these chemical unions.
The quintessential covalent bond is characterized by the sharing of electrons between two nonmetal atoms, igniting a journey where they transform into molecules. The allure of covalent compounds lies in their versatility. With a multitude of arrangements and interactions, they can exist as gases, liquids, or solids, each state offering a unique experience, much akin to the way different musicians contribute to a symphony, creating a rich auditory experience.
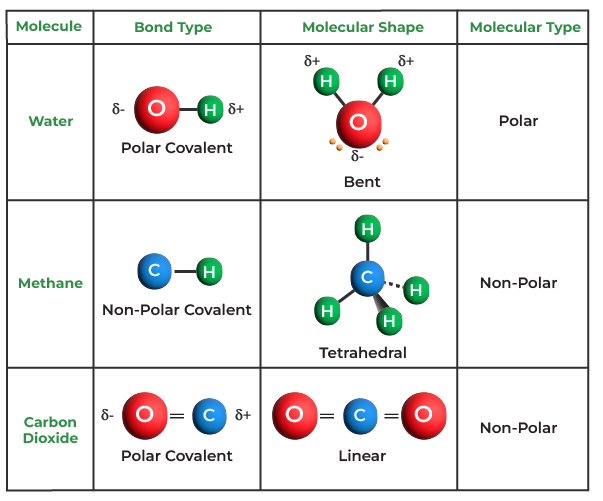
One illustrious example of a covalent bond compound is water, or H2O. This modest molecule is truly extraordinary. Picture two hydrogen atoms, akin to eager dancers, orbiting around a more substantial oxygen partner. Their union is a perfect illustration of harmony; as they share electrons, water emerges as a universal solvent, pivotal for life. It has a strange but appealing trait—its solid form, ice, is less dense than its liquid counterpart. This seemingly whimsical characteristic enables ice to float, becoming a protective blanket for aquatic life during frigid winters.
Another exciting covalent compound to explore is carbon dioxide, or CO2. In this arrangement, a carbon atom confidently wields its four available electrons, forming two robust double bonds with two oxygen atoms. This molecule, often perceived merely as a greenhouse gas, is crucial in the grand cycle of life. Through photosynthesis, plants absorb carbon dioxide and, in a miraculous turn, release oxygen, offering sustenance to aerobic organisms. This symbiosis showcases the unity found within covalent bonding; it underlines the interdependence of various life forms, akin to a tightly-knit community, where each member relies on the others to thrive.
Moving deeper into the covalent treasury, let us examine methane (CH4). Often dubbed the simplest organic compound, methane consists of one carbon atom bound to four hydrogen atoms, resembling a tetrahedral arrangement akin to a pyramid. It is primarily associated with natural gas—an energy powerhouse. Methane serves as a pristine source of fuel, efficiently converting chemical energy into thermal energy. Its structure is not just appealing but ingeniously designed; the symmetry of methane allows it to pack densely, resulting in a high-energy yield per molecule, making it a linchpin in the energy sector.
As we explore more complex covalent compounds, let us not overlook ethylene (C2H4), a small hydrocarbon molecule that plays a pivotal role in the world of botany and agriculture. With its double bond between two carbon atoms, ethylene is instrumental in the ripening process of fruits. Fruits produce ethylene in response to certain stimuli, and this gaseous hormone encourages ripening, altering texture and flavor, resembling the gradual unfolding of a flower’s petals in the warm embrace of the sun. Beyond its role in the natural world, ethylene serves as a precursor in the production of polymers, showcasing its industrial significance as well.
Then comes the illustrious world of amino acids, the building blocks of proteins. Each amino acid is distinguished by its side chain, but the peptide bonds that link them are formed through covalent bonding. These bonds allow for the intricate folding patterns required for proteins to function, akin to a finely crafted origami piece that transforms a flat sheet into a complex form with utility and beauty. Proteins, forged by the covalent bonds of amino acids, underpin nearly every biological process, making this interaction a cornerstone of molecular biology.
To truly appreciate covalent bonds, one must acknowledge their display of duality. On the one hand, they can form relatively weak van der Waals forces, creating transient yet vital interactions, while on the other, they yield strong covalent links that provide the backbone to robust molecular frameworks. This duality inspires awe; it illustrates the balance between fragility and strength, much like a delicate spider’s silk that can withstand the weight of rain without breaking.
As we contemplate the world of covalent bond compounds, it becomes clear that these chemical relationships are shaping the very fabric of life and industry. The very water we drink, the natural gas that warms our homes, and the proteins that enable our biological processes are but a few examples of the wonders birthed from the sharing of electrons. They remind us that at the molecular level, everything is connected, intertwined in a dance that transcends mere chemistry—an emblem of unity and collaboration in the ever-complex web of existence.
In conclusion, covalent bonds and their resulting compounds offer not just utility, but an intriguing perspective on the interrelatedness of life itself. They encapsulate a reality where shared resources create greater possibilities, fostering an environment ripe for growth and innovation. As we continue to unravel the complexities of chemistry, the lessons we learn from these bonds echo beyond laboratories and classrooms, resonating in our everyday experiences.