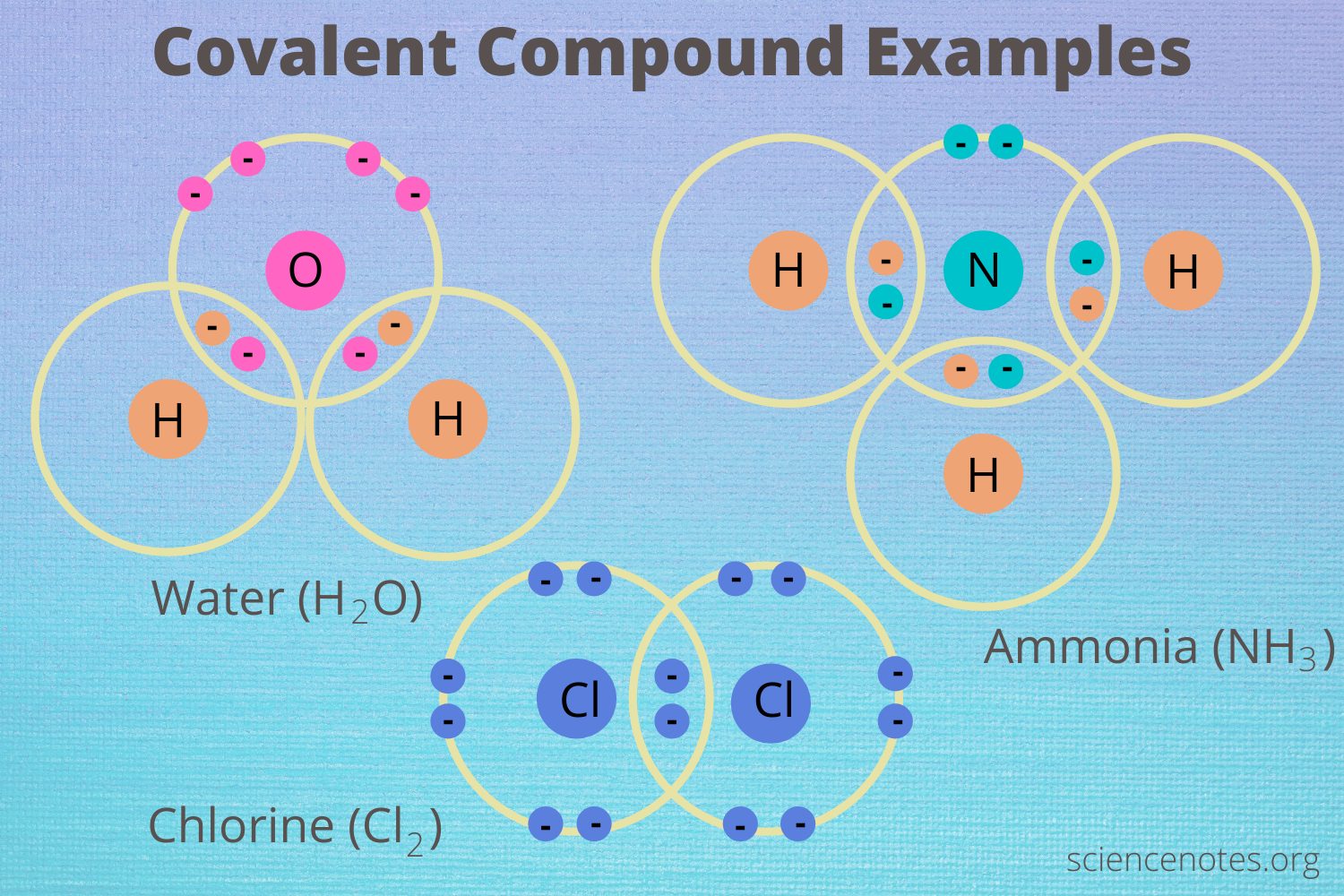
Covalent compounds are a fascinating category of substances that possess unique characteristics, underscoring the complexity of chemical interactions. Unlike ionic compounds, which are typically formed through the donation and acceptance of electrons, covalent compounds are created through the sharing of electron pairs between atoms. This shared relationship results in the formation of molecules that exhibit an array of behaviors and properties, making them crucial in both chemistry and the broader scope of life itself.
One of the most illustrative examples of a covalent compound is water (H2O). This simple yet vital molecule showcases the quintessential properties of covalent bonds. Each water molecule consists of two hydrogen atoms covalently bonded to a singular oxygen atom. The electrons are shared unequally in this arrangement, resulting in a polar molecule where one side has a slight positive charge while the other has a slight negative charge. This polarity not only confers notable properties to water, such as its high surface tension and excellent solvent capabilities, but it also plays an indispensable role in supporting life.
Delving deeper into the intricacies of water, one cannot overlook its ability to exist as a liquid over a wide range of temperatures. This phenomenon is largely due to the network of hydrogen bonds formed between water molecules. These bonds, though weaker than covalent bonds, significantly contribute to water’s high boiling and melting points when compared to other similar-sized molecules. The heat capacity of water, which allows it to absorb and store heat, is another attribute profoundly influencing climate and ecosystems worldwide. It is the coherent dance of covalent bonding and non-covalent hydrogen interactions that endows water with its unparalleled properties.
Beyond water, carbon dioxide (CO2) presents another significant example of a covalent compound. Formed by the sharing of electrons between one carbon atom and two oxygen atoms, carbon dioxide is a linear molecule with a nonpolar configuration. Its role as a greenhouse gas in the Earth’s atmosphere is critically important. Although it represents a modest fraction of atmospheric gases, its contribution to the greenhouse effect is exacerbated by human activity, showcasing the intersection of covalent chemistry and environmental science. As CO2 levels rise, the implications for global temperatures and climate patterns signal a compelling need for scrutiny and action.
The molecular interactions represented in covalent compounds extend far beyond simple examples. Glucose (C6H12O6), a fundamental carbohydrate, embodies the complexity and significance of covalent bonding in biological systems. In its structure, glucose contains numerous covalent bonds that enable it to form various isomers, each with distinct properties and physiological roles. The versatility of glucose as an energy source in living organisms highlights its foundational importance to cellular processes, illustrating how covalent compounds can serve as the building blocks of life itself.
Moreover, the intricate world of organic chemistry further exemplifies the diverse applications of covalent compounds. Amino acids, the fundamental units of proteins, are another important category of covalently bonded substances. Each amino acid comprises an amino group, a carboxyl group, and a distinctive side chain, showcasing how varying covalent structures lead to an expansive variety of proteins, each with its unique function. The myriad of interactions—ranging from hydrogen bonds to disulfide bridges—between these compounds influences protein folding and functionality, shedding light on the delicate nature of life on a molecular level.
Another noteworthy covalent compound is methane (CH4), the simplest alkane and a primary component of natural gas. Methane is formed when a carbon atom shares its electrons with four hydrogen atoms, resulting in a tetrahedral geometry. This compound is infamous for its role as a potent greenhouse gas, with its global warming potential significantly overshadowing that of carbon dioxide over a shorter time frame. The duality of methane—serving as a vital energy resource while simultaneously presenting environmental challenges—illustrates the complex interplay between human enterprise and ecological stewardship.
Due to their unique properties, covalent compounds are not only commercially significant but also pose intriguing scientific inquiries. For instance, exploring the behavior of covalent compounds at varying temperatures often leads to discoveries about phase transitions and material properties. Understanding how the strength of covalent bonds translates to hardness and melting points is critical in fields ranging from materials science to nanotechnology. Such explorations embody the scientific method’s essence: deriving insights from observed phenomena and seeking to elucidate the underlying principles that govern them.
The study of covalent compounds also serves as a gateway to appreciating the omnipresent nature of chemistry in our daily lives. From the food we consume, consisting of proteins and carbohydrates, to the air we breathe, containing essential gases like oxygen and carbon dioxide, the influence of covalent bonding is ubiquitous. The advancements in covalent compound synthesis continue to expand our arsenal of materials, fuels, and pharmaceuticals, ultimately driving progress in technology and healthcare.
In conclusion, covalent compounds, exemplified by simple yet profound molecules such as water, carbon dioxide, glucose, and methane, represent a cornerstone of chemistry and biology. Their distinctive characteristics not only illustrate fundamental chemical principles but also point to the intricate interdependencies that sustain life on Earth. The exploration of these compounds propels us toward a deeper understanding of our world and reinforces the importance of chemistry as a science that continually shapes our existence in myriad ways.