Combustion is a fundamental chemical reaction that occurs when a substance reacts with oxygen, producing heat and light. This intricate dance of molecules not only plays a critical role in our everyday lives, but it also underpins many industrial processes. Understanding combustion reactions invites us to reevaluate the nature of energy transfer and the transformations that fuel our world. This article delves into the intriguing realm of combustion reactions, exploring their types, mechanisms, importance, and real-life examples that underline their significance.
1. The Essence of Combustion: A Brief Overview
At its core, combustion encompasses an exothermic reaction between a fuel and an oxidant, typically oxygen. This reaction can be exemplified by the burning of hydrocarbons, where organic compounds composed primarily of carbon and hydrogen undergo an oxidation process. When these molecules encounter sufficient oxygen, a release of energy occurs in the form of heat and light, often witnessed as flames. However, not all combustion reactions yield flames; smoldering or glowing combustion can also exist, signifying the presence of insufficient oxygen or a lower reaction rate.
2. Types of Combustion Reactions
Combustion reactions can be categorized into several distinct types, each characterized by its unique features:
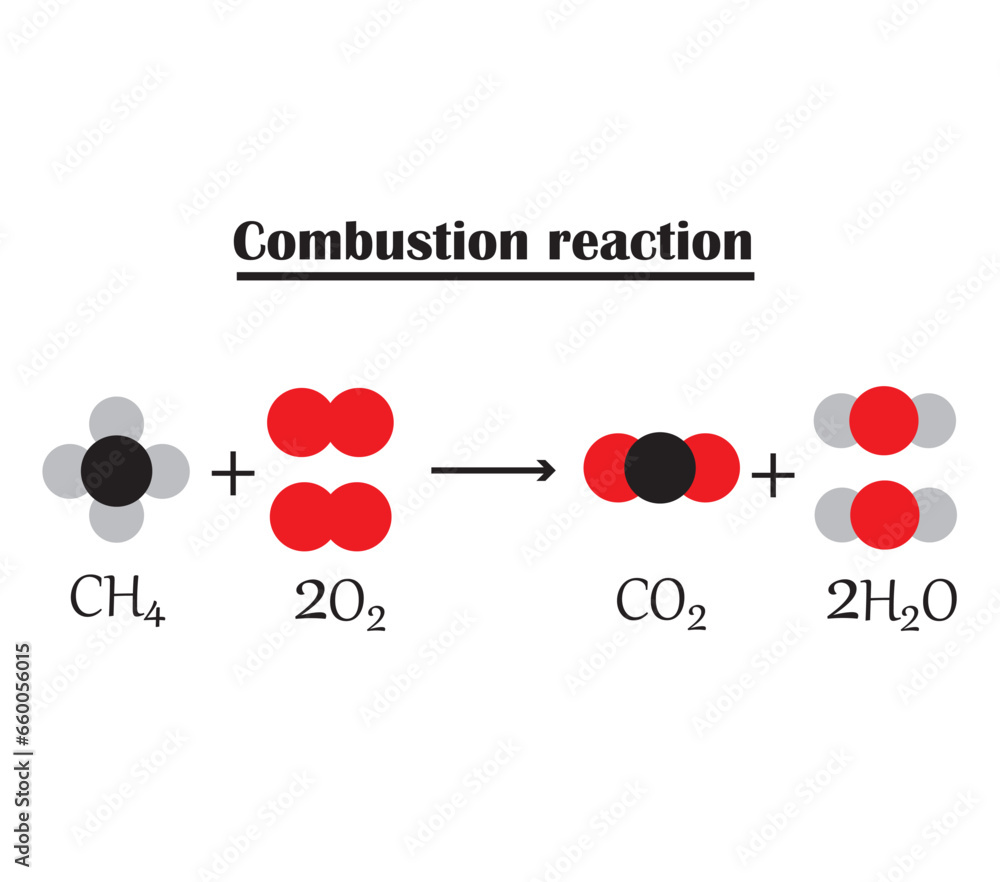
- Complete Combustion: This occurs when a fuel burns in ample oxygen supply, resulting in carbon dioxide and water as products. For instance, the complete combustion of methane (CH4) occurs as follows:
CH4 + 2O2 → CO2 + 2H2O
2CH4 + 3O2 → 2CO + 4H2O
3. Significance of Combustion Reactions
The ramifications of combustion extend far beyond mere flames; they are integral to various domains. The energy produced from combustion drives vehicles, heats homes, and powers industries. Electric power generation heavily relies on combustion, particularly coal and natural gas. Furthermore, the beauty of combustion extends to the culinary world. The Maillard reaction, linking the browning of food to complex chemical transformations during cooking, is a delightful example of combustion’s culinary application.
4. Interesting Examples of Combustion Reactions
Exploring the universe of combustion leads us to fascinating and illustrative examples:
- The Burning of Natural Gas: Natural gas, primarily composed of methane, is a prevalent fuel source. Its combustion is not only efficient but also cleaner relative to other fossil fuels. The reaction produces a blue flame, a hallmark of complete combustion, as illustrated in household heating systems and stoves.
- Fires in Nature: Forest fires may initiate through natural causes, such as lightning strikes. These uncontrolled combustive events illustrate the fragility of ecosystems and the duality of combustion, acting as both destroyer and renewer of habitats.
- Candles: The serene flicker of a candle flame represents a quintessential combustion reaction. As the wax evaporates, it reacts with oxygen, producing water vapor and carbon dioxide, along with the warm glow that enhances intimate environments.
- Propellant Combustion in Rockets: Rocket engines employ combustion reactions to achieve thrust. The intense combustion of fuel generates superheated gases expelled at high speed, enabling spacecraft to breach Earth’s atmosphere, a stellar demonstration of Newton’s Third Law in action.
- Internal Combustion Engines: Found in most vehicles, these engines convert fuel into mechanical energy through a series of controlled combustion events. The efficiency of this process is a blend of chemistry and engineering, propelling society forward.
5. The Chemistry of Combustion
Diving deeper into the chemical underpinnings, combustion reactions typically involve a fuel, an oxidizer, and temperatures high enough to initiate the reaction. The progress of combustion reactions is influenced by various factors, including the fuel’s structure, environmental conditions, and the presence of pollutants. The stoichiometry, or the quantitative relationship between reactants and products in a combustion reaction, is critical in determining the efficiency and cleanliness of the process.
Conclusion
Combustion reactions are a remarkable intersection of chemistry and real-world application, shaping the foundation of energy production, environmental dynamics, and culinary arts. By exploring their types, significance, and examples, we cultivate a newfound appreciation for the role they play in our daily existence. As technology advances and energy needs evolve, the science of combustion continues to challenge our understanding and expand the horizons of innovation. From the warmth of a candle flame to the thrust of a rocket, the story of combustion is both profound and ever-relevant.