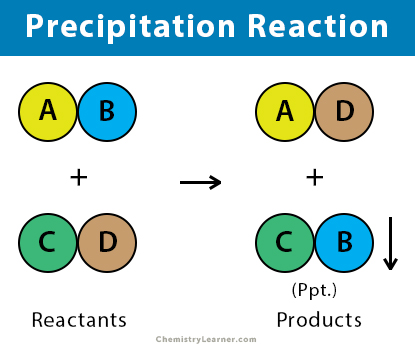
Have you ever wondered how some substances can appear from nothing, seemingly materializing in a dissolved solution? This phenomenon, known as a precipitation reaction, transforms soluble reactants into an insoluble solid called a precipitate. But why does this happen? Let’s delve into some riveting examples of precipitation reactions that illuminate this intriguing aspect of chemistry.
To truly appreciate precipitation reactions, it is essential to grasp their underlying principles. In essence, a precipitation reaction occurs when two aqueous solutions, containing ions, are mixed, leading to the formation of an insoluble compound. This process often results in a striking visual transformation, where the precipitate might manifest as a vibrant color contrasting sharply with the clear solution. The notion of solubility plays a pivotal role here—ions that remain in solution are soluble, whereas those that combine to form a precipitate are inherently insoluble under given conditions.
Now, let’s explore some classic examples that illustrate the remarkable nature of precipitation reactions.
Example 1: Barium Sulfate Formation
One notable example of a precipitation reaction is the formation of barium sulfate (BaSO4). When barium chloride (BaCl2) is mixed with sodium sulfate (Na2SO4), barium sulfate precipitates out of the solution. The balanced chemical equation for this reaction is:
BaCl2 + Na2SO4 → BaSO4 (s) + 2 NaCl
Here, barium sulfate presents as a white solid, showcasing the characteristic appearance of many precipitates. This reaction is particularly significant in medical diagnostics and environmental chemistry, where barium sulfate is employed in X-ray imaging as a radiopaque contrast agent.
Example 2: Silver Chloride Production
Another illustrative example involves the formation of silver chloride (AgCl). When solutions of silver nitrate (AgNO3) and sodium chloride (NaCl) are combined, a beautiful white precipitate of silver chloride emerges:
AgNO3 + NaCl → AgCl (s) + NaNO3
This reaction vividly highlights the instantaneous formation of a precipitate, which can also appear as fine white particles suspended in the liquid. Silver chloride has noteworthy applications in photography and in the manufacture of certain types of sensors.
Example 3: Lead(II) Iodide Formation
Lead(II) iodide (PbI2) serves as a quintessential example of a brightly colored precipitate. When lead(II) nitrate (Pb(NO3)2) reacts with potassium iodide (KI) in an aqueous solution, we observe the formation of a brilliant yellow precipitate:
Pb(NO3)2 + 2 KI → PbI2 (s) + 2 KNO3
This reaction not only produces visually stunning results but also has significant implications in education, where it serves as an engaging demonstration of precipitation reactions in the laboratory setting.
Understanding Factors Influencing Precipitation
Several factors influence the occurrence and extent of precipitation reactions. Concentration plays a pivotal role; higher concentrations of reactants typically enhance the likelihood of a precipitation reaction. Temperature can also be a factor. In some cases, increasing the temperature may favor the solubility of certain salts, effectively suppressing precipitation.
Additionally, pH can dramatically influence these reactions. For instance, certain precipitates are more likely to form in acidic or basic conditions. By manipulating the environmental conditions, chemists can control the precipitation to their advantage.
Real-world Applications of Precipitation Reactions
Precipitation reactions extend far beyond mere classroom demonstrations. In wastewater treatment, for instance, precipitation methods are employed to remove unwanted ions. By adding specific reagents, pollutants can be transformed into insoluble precipitates, effectively cleaning the water.
In the realm of pharmaceuticals, precipitation reactions are crucial for synthesizing various compounds, ensuring purity and proper dosage in medications. Furthermore, in the field of materials science, precipitation is utilized in the creation of nanoparticle composites, which have significant applications in electronics and nanotechnology.
The Challenge of Solubility Rules
Consequently, mastering these rules not only enhances one’s understanding of chemistry but also hones analytical thinking skills. It invites inquisitive minds to explore the intricate details of ionic interactions and reactions.
In conclusion, precipitation reactions showcase the fascinating world of chemistry, where appearances can be deceiving. From vivid colors to the transformation of solutions into solids, these reactions embody the marvels of science. Whether in a laboratory or the environment, understanding precipitation reactions opens the door to myriad applications, challenges, and questions that continue to captivate chemists and enthusiasts alike.