When one gazes upon the myriad forms of matter in our universe, the solidity of certain substances stands out prominently. The solid state of matter is perhaps the most familiar phase we encounter daily, yet its intricate nature and profound implications often remain enigmatic. This article delves into the characteristics of solids, the diverse types that exist, and their ubiquitous presence in our lives, shedding light on why they captivate our imagination.
Defining Characteristics of Solids
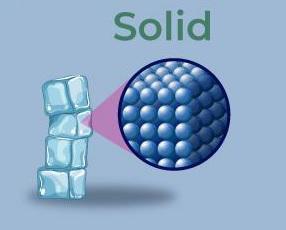
To comprehend the allure of solid matter, one must first explore its defining characteristics. Solids are distinguished by their definite shape and volume, a stark contrast to the fluidity of liquids and the expansiveness of gases. At the microscopic level, the particles in a solid—atoms or molecules—are tightly packed in a structured arrangement, which engenders rigidity and incompressibility. This structural density allows solids to maintain their shape even under substantial pressure.
Further entrenching their fascinating nature, most solids exhibit an ability to withstand deformation up to a certain limit, referred to as elasticity. When a force is applied, solids may bend or compress, but they often return to their original form once the stress is removed. This unique property has profound implications in engineering and materials science, allowing for innovation in design and architecture.
Solid States: Crystalline and Amorphous
Within the realm of solids, two primary classifications exist: crystalline and amorphous solids. Crystalline solids, such as diamonds and table salt, possess a highly ordered structure where the constituent particles are arranged in a repeating lattice. This symmetry does not merely confer aesthetic appeal; it also dictates the material’s melting point, optical properties, and electrical conductivity.
Conversely, amorphous solids, such as glass and many plastics, lack this orderly arrangement. In these substances, particles are arranged randomly, akin to a disorganized collage. While they possess the ability to maintain shape, their lack of long-range order results in different thermal and mechanical behaviors compared to their crystalline counterparts. This divergence sparks intrigue and presents various applications across industries.
Everyday Examples of Solid Matter
Consider the everyday objects that populate our environment—each often an embodiment of solid matter, yet vastly different in form and function. Take, for example, metals like iron and aluminum, characterized by their malleability and ductility, allowing them to be shaped into myriad tools and structures. Such materials play an integral role in construction and manufacturing, showcasing the solid phase’s utility.
Wood, another quintessential solid, epitomizes the diversity found within this state. As a natural composite material, wood’s fibrous structure lends strength and longevity while being relatively lightweight. It has been a staple in construction and craftsmanship for millennia, demonstrating the balance between aesthetic beauty and structural integrity.
Moreover, one cannot overlook the significance of solid-state materials in the modern technological landscape. Semiconductors, often crystalline in nature, are fundamental to electronics, enabling the revolution of communication and information technology. The transition from solid particles in dense structures to the microscopic worlds of electronic conductivity accentuates the pivotal role of solids in shaping contemporary life.
Solid State in Nature: A Window into Our World
The fascination with solid matter extends into the natural realm as well, where processes like crystallization reveal nature’s artistry. For instance, the formation of snowflakes, each a unique crystalline structure, invites admiration for the complexity underlying what might seem superficially simple. These tiny, intricate formations are the result of atmospheric conditions—freezing temperatures and humidity—culminating in spectacular unique patterns that echo the fantastic order of crystalline solids.
Similarly, the geological marvel of minerals, solid crystalline structures formed over millennia, captivates our curiosity. From the dazzling allure of quartz to the rich hues of malachite, these minerals not only adorn our planet but also hold keys to understanding its history and the processes that have shaped it. The geological narrative contained within these solid forms speaks to a deeper connection between the physical world and its historical context.
The Role of Solids in Science and Innovation
Beyond their aesthetic and functional roles, solids continue to play a central part in scientific research and innovation. The study of solid-state physics and materials science is an expansive field, aimed at understanding how solid materials behave under various conditions. Researchers tirelessly explore novel materials that exhibit extraordinary properties, such as superconductors and quasicrystals, potentially unlocking new frontiers in technology.
Moreover, the quest for sustainable materials has garnered significant attention, leading to the development of biodegradable solids that can reduce environmental footprints. Innovations in polymer science yield materials that blend functionality with ecological responsibility, resulting in a fascinating intersection of chemistry and environmental stewardship.
Conclusion: The Enduring Fascination with Solids
The solid state of matter embodies a confluence of beauty, complexity, and utility that resonates across various disciplines and everyday life. From the striking forms of natural minerals to the engineered precision of modern materials, solids provoke reflection on their multifaceted roles in our world. As we unravel the intricacies of these substances, we also unveil the broader principles that govern nature—an exploration that continues to ignite curiosity and drive discovery. In recognizing the myriad manifestations of solid matter, we touch upon deeper questions about our universe and our place within it, reminding us that even the most commonplace substances are steeped in wonder.