Imagine a world where atoms dance a delicate tango, their charged partners drawn together by the invisible forces of nature. This captivating interplay is the essence of the ionic bond, a fundamental concept in chemistry that binds an array of elements in a stunning display of attraction and repulsion. But what exactly is an ionic bond, and how does it manifest in the realm of chemical compounds? Let’s delve into the intricacies of this chemical relationship by exploring a vivid picture example of an ionic bond.
At its core, an ionic bond forms between two atoms: one that readily relinquishes electrons and another that eagerly accepts them. This transfer of electrons leads to the formation of charged particles known as ions. The element that donates electrons transforms into a positively charged cation, while the recipient becomes a negatively charged anion. The electrostatic attraction between these oppositely charged ions results in a stable compound, typically characterized by an impressive array of properties.
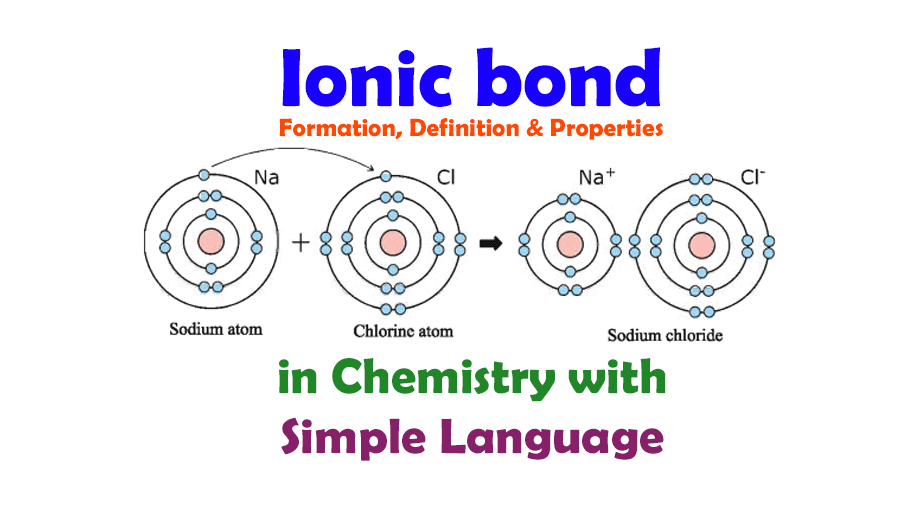
To visualize this phenomenon, consider a classic example: sodium chloride (NaCl), commonly known as table salt. Picture a sodium atom, gleaming like a beacon of energy, situated in group one of the periodic table. It possesses a single electron in its outermost shell, a gift it yearns to part with. Now, juxtapose this with a chlorine atom, perched in group seventeen, just one electron shy of achieving a complete octet. What if, with a dramatic flourish, our sodium atom relinquishes its solitary electron to its chlorine counterpart? The result is a stunning transformation.
Once sodium sheds its electron, it morphs into a positively charged Na+ ion. Meanwhile, the chlorine atom, having accepted the electron, transforms into a negatively charged Cl– ion. The moment these two ions come together, a powerful attraction ignites, forming what we recognize as an ionic bond.
Wouldn’t it be fascinating to visualize this electron-transfer process? Let’s think of it as a game of tag where sodium generously hands over its electron to chlorine, making both participants more stable in the process. This playful interaction is not only fundamental to chemistry but is also essential for understanding how substances like salt are structured.
As we navigate through the components of an ionic bond, it is crucial to appreciate the resulting lattice structure formed when numerous ions come together. This ionic lattice is a compelling aspect of ionic compounds, characterized by ions arranged in repetitive three-dimensional patterns. The robust nature of these structures accounts for several notable properties of ionic compounds.
For instance, ionic compounds typically have high melting and boiling points. This phenomenon results from the strong electrostatic forces that hold the ions together within the lattice. It implies that a significant amount of energy is required to break these bonds, making it difficult to melt or boil such compounds. Think about it this way: just as a fortress needs a colossal battering ram to fracture its gates, ionic compounds resist changes in temperature and state.
Additionally, those distinctive properties extend to solubility. Many ionic compounds dissolve readily in water, a trait attributed to the polar nature of water molecules. Imagine the atoms relinquishing their ordered dance within the lattice; they enter the fluid world of water, allowing their individual ions to roam free. The moment sodium chloride is introduced to water, it disassociates, permitting the cations and anions to disperse uniformly throughout the solution. This is why salt dissolves so seamlessly in water, an effect that has profound implications in culinary practices and various industrial applications.
Another intriguing aspect to consider is electrical conductivity. In their solid state, ionic compounds do not conduct electricity; the ions are locked in place within their rigid lattice structure. However, once dissolved in water or melted into a liquid state, the ions gain the freedom to move. The resulting solution or molten substance becomes a conductor of electricity, illuminating the pathways of electrical currents—a concept vital for numerous technological applications.
But what about the visual representation of an ionic bond? An impactful picture can crystallize these concepts perfectly. For instance, an illustration depicting the transfer of an electron from sodium to chlorine, with arrows indicating the flow and transformation, serves as both an educational tool and a point of curiosity. Envision a vibrant graphic where the ions are depicted in contrasting colors, symbolizing their positive and negative charges. Such imagery provides a striking visual metaphor for understanding ionic bonding.
In conclusion, ionic bonds serve as foundational elements in the chemical universe, connecting diverse elements in remarkable ways. They demonstrate how the simple act of one atom giving away an electron can lead to the creation of stable compounds with distinct properties. A picture speaks a thousand words, especially when it dramatically illustrates the bond between sodium and chlorine, showcasing the beautiful chaos of atomic interaction. With this understanding, one can appreciate the broader implications ionic bonds have in the material world— from the salty delights on our dinner tables to the very essence of life itself.
As you reflect upon the concept of ionic bonding, consider your own interactions and connections. Are you forging bonds that enhance your stability? Or perhaps there’s an electron—an idea or opportunity—that you’re ready to share? The dance of life, much like the dance of atoms, is one of give and take.